Hopeful results in latest HIV vaccine trial, but many hurdles to overcome yet
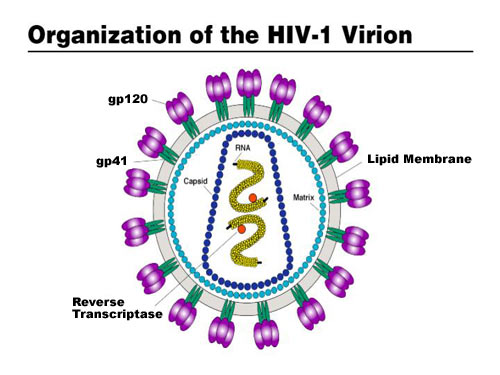
A HIV vaccine, known as SAV001-H has shown promising results in an early clinical trial, with no adverse effects reported and importantly, a significant increase reported in HIV specific antibodies in participants who received the vaccine. In this trial, 33 HIV positive participants were randomly allocated to one of two groups: half into a treatment group receiving the vaccine and half into a placebo group who did not receive the vaccine. The participants were followed up at regular periods, testing safety of the vaccine and antibody response over a one year period. The vaccine has been developed by Dr C. Yong Chan and his team at the University of Western Ontario and has been licensed for commercialisation by the biotechology company Sumagen.
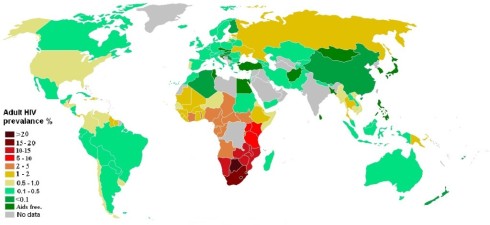
This is welcome news for a disease which killed an estimated 1.7 million people in 2011 (WHO, 2013). Furthermore, there are an estimated 34 million people around the world living with HIV/AIDS, and the disease continues to have a devastating impact in Sub-Saharan Africa where up to a quarter of the population in countries such as Botswana, Lesotho and Swaziland are infected (WHO 2013; UNAIDS 2013). While great strides in treatment of HIV; via the development of antiretroviral (ART) medications, have been made by extending the time it takes for HIV to develop to AIDS, ART treatment is not yet available to all, particularly in the poorest nations; and of course, treatment is much less optimal then prevention of HIV in the first place.
The long road to effective HIV vaccines
However development of successful HIV vaccines has proven to be highly elusive, due in part to the rapid mutation rate of HIV, these mutations generating a diverse population of quasi-species of HIV over the length of the infection period – the immune system, itself the target of HIV, is simply not able to keep up (Ackerman and Alter, 2013). This immense genetic diversity of HIV has meant that targeting HIV with effective, universal vaccines has been particularly difficult.
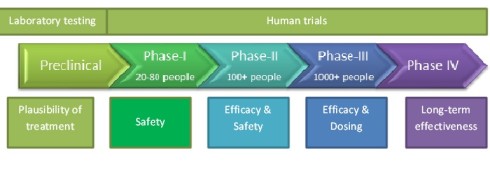
This leads to a cautionary note that must be taken regarding SAV001-H; this was a small, very early trial of this particular HIV vaccine. These early trials are referred to as Phase 1 trials, meaning that its primary focus was on assessing the safety of vaccine, with efficacy or effectiveness assessed only as a secondary objective (see graphic, below). Phase 2 and 3 trials are planned next and it is these trials which are particularly focused on assessing the effectiveness of the vaccine to prevent HIV infection in large numbers of HIV negative participants at high risk of contracting HIV. Furthermore, other promising HIV vaccines have passed through early Phase 1 trials, only to show in later trials no significant efficacy. An example of this was a highly anticipated STEP trial, which was a large Phase 2 trial enrolling 3,000 high risk individuals randomised into a vaccine or placebo group. The vector based vaccine used in this trial failed to show any effect on reducing risk of infection and indeed those receiving the vaccine appeared to be at greater risk of contracting HIV (HTNV, 2013).
Thus some of the early media reports for SAV001-H which have been mentioning ‘eradication’ are very premature and paying scant heed to the long (and sometimes disappointing) road that this vaccine must pass through with its coming Phase 2 and 3 trials, as demonstrated above with the example of the STEP HIV vaccine trial. These promising early results from this latest HIV vaccine trial must be tempered by the historical difficulties in conducting successful HIV vaccine trials.
Cautious optimism
Despite the very real need for caution at this early stage, there are several reasons why many in the health community are particularly optimistic about SAV001-H. This optimism is largely due to the fact that SAV001-H takes a new approach to HIV vaccine design as it uses whole killed HIV viruses. Previous HIV vaccine strategies have used other strategies such as subunit vaccines which basically introduce important proteins (called antigens) to the body to induce a specific immune response, or vector based vaccines to introduce genetic material from the HIV virus via another ‘carrier’ virus; both approaches proving disappointing so far (Sumagen, 2013).
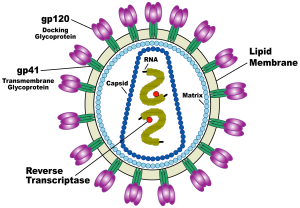
For SAV001-H, the HIV-1 virus is genetically engineered by deleting the activity of specific genes involved in the disease causing process (pathogenicity) and then chemically treated and bombarded with gamma radiation to disable its ability to multiply within human cells (virulence) (Sumagen, 2013). This way, the immune system will still detect and mount a response against the invading virus, but the virus is no longer able to cause disease. The early data from the Phase 1 trial are particularly encouraging as it has been reported that vaccination with SAV001-H produced large increases in two particular antibodies specific for the p24 envelop antigen and gp120 surface antigens of HIV-1 (Western News, 2013).
While gaining plenty of attention, SAV001-H is only one of multiple vaccines in development; a major focus of several other research efforts, are vaccines designed to induce potent antibodies, known as broadly neutralizing antibodies (bNAbs), that are able to potentially block HIV infection (Korber and Gnanakaran, 2011). Promising early research into vaccines that may be able to effectively induce these bNAbs and provide effective immunity against HIV infection have provided another boost to HIV vaccine development efforts. So there is certainly reason to be hopeful about SAV001-H, but we need to mix this hope with a healthy dose of caution at this stage. Also, it is worth noting that any effective HIV vaccines will likely be just one, albeit very important, part in a multitude of preventative strategies, including sex education, male circumcision, microbiocide gels and prophylactic antiretroviral therapies which will result in the effective prevention of HIV infections worldwide.
Sources:
1. World Health Organisation. HIV Data and Statistics [Online]. Available at: http://www.who.int/hiv/data/en/
2. United Nations AIDS (UNAIDS). AIDS Information by country [Online]. Available at: http://www.unaids.org/en/dataanalysis/datatools/aidsinfo/
3. Ackerman M, Alter G. 2013. Mapping the Journey to an HIV Vaccine. NEJM 369(4): 389-391.
4. HIV Vaccine Trials Network (HVTN) [Online]. Available at: http://www.hvtn.org/science/step_buch.html
5. Sumagen. AIDS vaccine [Online]. Available at: http://www.sumagen.co.kr/english/business/aids_vaccine.htm
6. HIV vaccine produces no adverse effects in trials. Western News, September 3, 2013 [Online]. Available at: http://communications.uwo.ca/western_news/stories/2013/September/hiv_vaccine_produces_no_adverse_effects_in_trials.html
7. Korber B, Gnanakaran S. 2011. Converging on an HIV Vaccine. Science 333; 1589-1590.
All images sourced from public domain (Wikimedia)
Borradale D (2013-09-23 00:24:51). Hopeful results in latest HIV vaccine trial, but many hurdles to overcome yet. Australian Science. Retrieved: Feb 24, 2026, from https://ozscience.com/health/hopeful-results-in-latest-hiv-vaccine-trial-but-many-hurdles-to-overcome-yet-3/
 Follow
Follow
2 thoughts on “Hopeful results in latest HIV vaccine trial, but many hurdles to overcome yet”
Comments are closed.